Now the enriollment for Regulatory Summit 2024 has opened! Join to hear Peter Löwendahl speak regarding several different topic. Read more here (note link in Swedish)
Regulatory summit
Events we speak at Posted on Dec 20, 2023 14:29:39- Comments(0) https://news.lowendahl.eu/?p=181
- Share
We speak at PLM forum October 21, 2021
Events we speak at, MDR/EU related, Regulatory Affairs Posted on Oct 20, 2021 12:38:47Come and listen to us but also many other comeptent speakers at the 2021 PLM forum. For more details and how to secure your access, visit the event page!
The title of our presentation is – Wake Up and Smell the Coffee!
Learn more about the challenges posed by Medical Device and In Vitro Medical Device Regulations. Discover how to manage this additional regulatory complexity by improving product documentation. And at the same time make this to be your additional competetive edge

- Comments(0) https://news.lowendahl.eu/?p=130
- Share
Egentillverkning IVDR och MDR 1 december 2021
Events we speak at Posted on Oct 19, 2021 12:24:30Vad gäller för vårdgivares egentillverkning av IVD och medicintekniska produkter samt hur är tillverkarens syn på detta! Detta och lite mer kommer att disskuteras om på detta event där vi pratar om tillverkarens perspektiv! Tillsyns myndigheten för detta område IVO kommer även att delta! Läs mer och anmäl er här

- Comments(0) https://news.lowendahl.eu/?p=121
- Share
Vi pratar på sterildagarna
Events we speak at Posted on Oct 19, 2021 12:10:04
To read more visit the website
- Comments(0) https://news.lowendahl.eu/?p=112
- Share
Strategi- och affärsutveckling för life science-ledare 20-22 oktober 2021
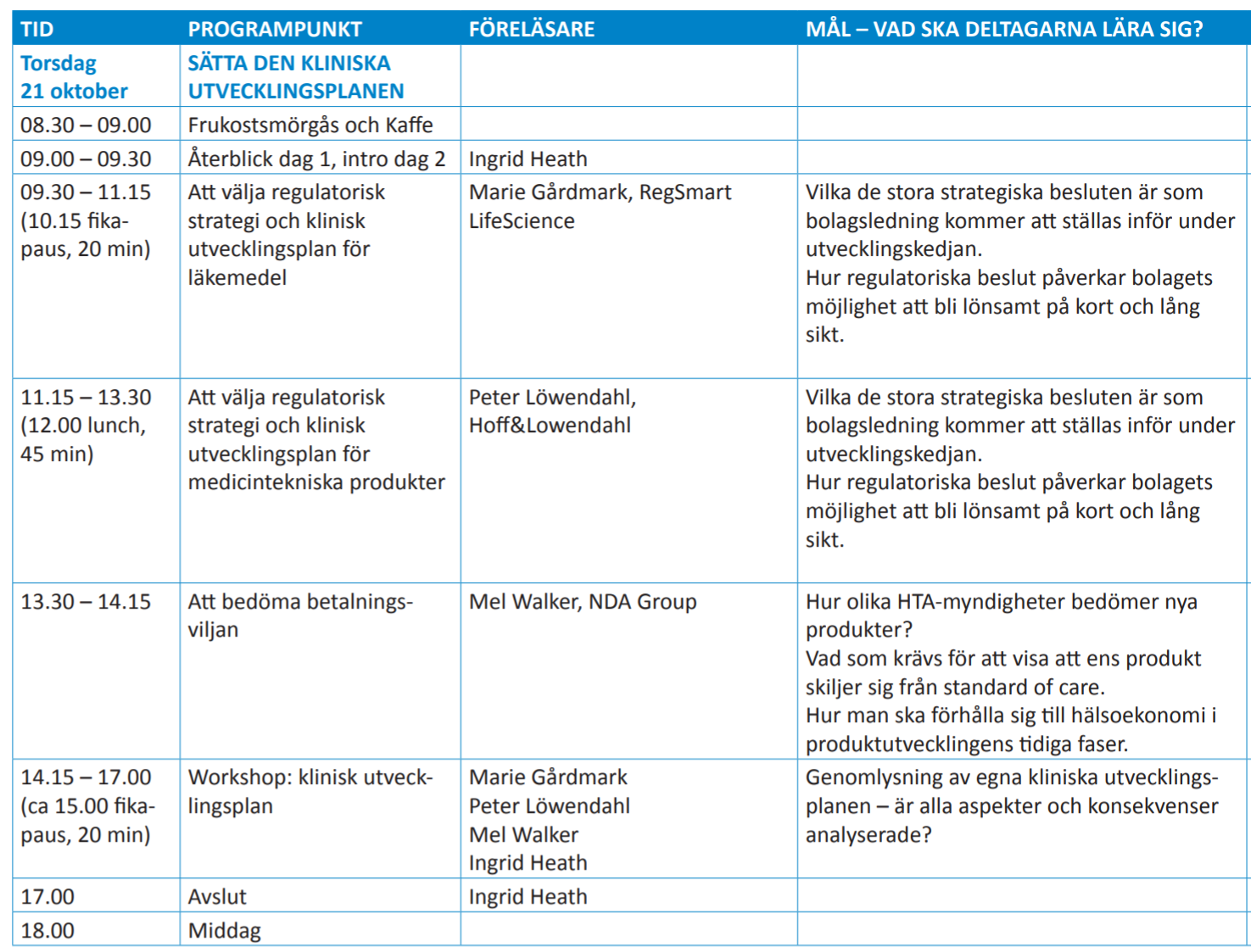
Events we speak at Posted on Sep 24, 2021 19:24:01I den här 4 dags kursen får ledare möjligheten att gå igenom allt från ax till limpa för ett företag. Vi kommer att föreläsa om och ha grupp arbeten runt de strategiska valen för medicintekniska produkter och hur man kopplar detta till den kliniska planen.
Fullständigt program hittar ni här. Men skynda att anmäla er, bara någon enstaka plats kvar!
Nedan extrakt från agendan från en av de 4 dagarna!
- Comments(0) https://news.lowendahl.eu/?p=109
- Share
Latest IVDR pod
Events we speak at Posted on Sep 24, 2021 12:16:30You can listen to the podd here. In this Podd Anna (CEO) at Swedish Medtech and Peter Löwendahl talks about the IVDR regulation and challenges for manufacturers to meet the requirements in time!
As stated before our view is that IVD companies need to get one more year to be able to get hold of a Notified Body.

- Comments(0) https://news.lowendahl.eu/?p=106
- Share
Medtech Pod
Events we speak at, Uncategorised Posted on Sep 20, 2021 19:10:48Swedish Medtech have several podds about medical devices in general but also a few specfic ones discussing the new regulations with Peter Löwendahl from Hoff&Lowendahl. These can be found here. Today we recorded a new Podd that will be released soon!
- Comments(0) https://news.lowendahl.eu/?p=103
- Share
Clinical evaluation training November 9, 2021
Events we speak at, Uncategorised Posted on Sep 15, 2021 21:31:54This 2 day course gives you deeper insight in clinical evaluation and clinical trials for medical devices. It will be online. Find out more about the course here.
- Comments(0) https://news.lowendahl.eu/?p=99
- Share
Training for PRRC 28 September 2021 and 18 November 2021
Events we speak at, Uncategorised Posted on Sep 15, 2021 20:00:40In cooperation with Swedish Medtech we invite you to participate in this popular training. We will go yhtough all aspects on responsibilities, cover differences between countries and what to look out for. Ensure to get your seat reserved today through this link. This training will be online.
- Comments(0) https://news.lowendahl.eu/?p=96
- Share
Day about PMS September 23, 2021
Events we speak at, Uncategorised Posted on Sep 15, 2021 19:56:09This seminar is about Post Market Surveillance. We will specific talk about how to meet the obligations in MDR and IVDR and not violate GDPR. For many manufacturer it might be a balance act to meet both at the same time. In the seminar the basic parts in the regulations will be reviewed and what you should think about!
Read more about the event and reserve your seat here. The seminar will be online.
- Comments(0) https://news.lowendahl.eu/?p=94
- Share

