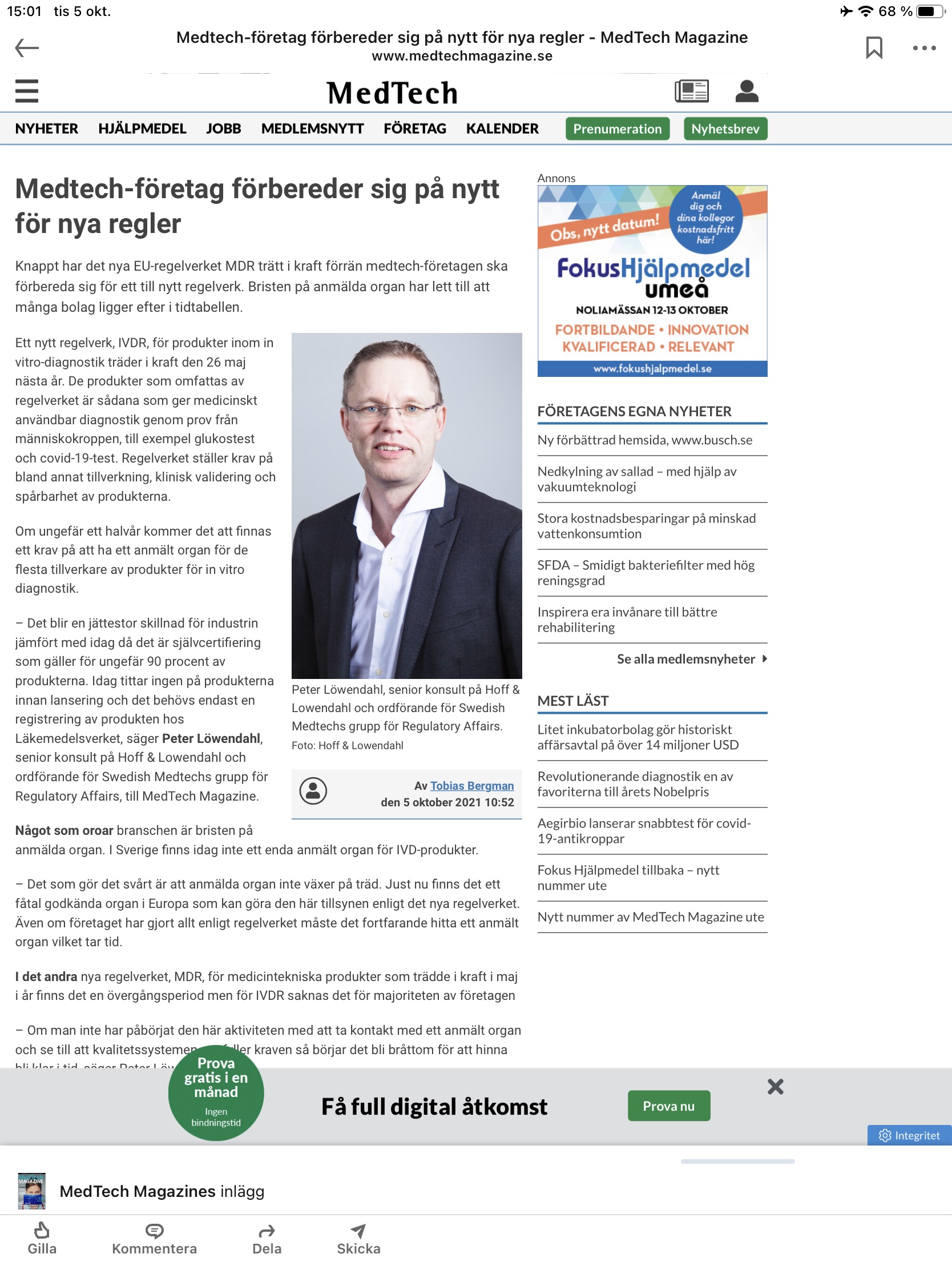
Our consultant Peter Löwendahl have been interviewed in the latest edition of Lifescience Sweden about IVDR and effects on the industry.
Read the article here
Our consultant Peter Löwendahl have been interviewed in the latest edition of Lifescience Sweden about IVDR and effects on the industry.
Read the article here
Sweden have now decided to allow reuse of non reusable medical devices! The decision and requirements can be found here.
We think this decision is the wrong one to take and could hevily effect patient safety. The sterilization methods used for reprocessing are for many type of diseases not enough. Typical example we have seen and experienced in real life are CJD.
Är du PRRC eller kommer att bli det enligt IVDR eller MDR? svarar du ja på den frågan är detta utbildningen för dig! Denna görs av oss i samarbete med Swedish Medtech!
Läs mer och anmäl dig här!

This years Regulatory Summit is taking place March 31, 2022. We will speak in several sessions.
Read more about it in this link. The plan this year is to have a hybrid meeting with both online and at location!
Ensure to grab your seat Today to listen to many good speakers abut very urgent subjects!

We will speak at this conference where buyers meets companies that provides medical devices!
Read more and secure your seat (zoom) here

Come and listen to us but also many other comeptent speakers at the 2021 PLM forum. For more details and how to secure your access, visit the event page!
Learn more about the challenges posed by Medical Device and In Vitro Medical Device Regulations. Discover how to manage this additional regulatory complexity by improving product documentation. And at the same time make this to be your additional competetive edge

Vad gäller för vårdgivares egentillverkning av IVD och medicintekniska produkter samt hur är tillverkarens syn på detta! Detta och lite mer kommer att disskuteras om på detta event där vi pratar om tillverkarens perspektiv! Tillsyns myndigheten för detta område IVO kommer även att delta! Läs mer och anmäl er här


To read more visit the website